ISO Changes: Managing Risk
The new ISO 9001:2015 standard emphasizes risk assessment. Medical shops with ISO 13485 certification already manage this.
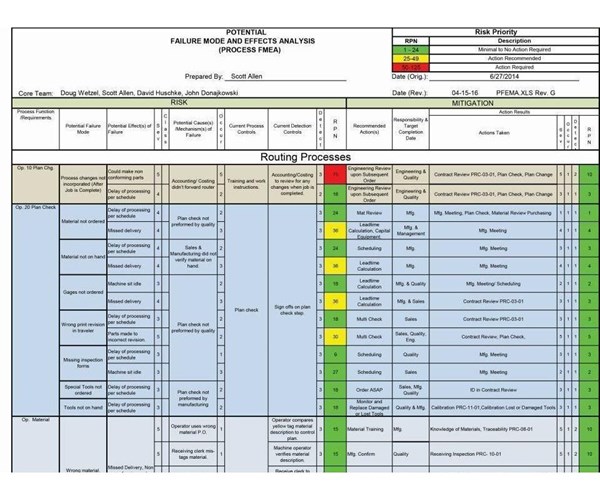
This is just a small portion of the process failure mode and effects analysis (PFMEA) document that Protomatic developed. It identifies more than 300 potential failure modes and offers procedures to mitigate those risks.
After the end of September 2018, a certificate to ISO 9001:2008 will no longer be valid. One change in its replacement ISO 9001:2015 is a requirement to establish procedures for comprehensive risk management. Shops with medical ISO 13485 certification already manage this.
Failure mode and effects analysis (FMEA) is one common method of risk identification and mitigation, although it is sometimes referred to differently from company to company. Protomatic, a Dexter, Michigan, prototype machining and custom short-run production facility serving the medical, automotive, military and aerospace industries, refers to its as PFMEA—process failure mode and effects analysis.
Learning about the risk management procedures this shop has put in place might serve as a good model for other, non-medical shops that will be working toward becoming registered to the updated standard. Learn more.
Related Content
-
Arch Cutting Tools Acquires Custom Carbide Cutter Inc.
The acquisition adds Custom Carbide Cutter’s experience with specialty carbide micro tools and high-performance burrs to Arch Cutting Tool’s portfolio.
-
Five-Axis Turnkey Machine Halves Medical Shop’s Cycle Times
Horizontal five-axis machines cut cycle times in half at ARCH Medical Solutions – Newtown. But its leadership gives equal credit to a surprising factor: the OEM’s service department.
-
EDM, Laser Micromachining and More at GF Medical Demo Center
At GF’s Medical Center of Competence, the company shows off EDM and laser features that could make a large impact on medical manufacturing — and elsewhere.

.jpg;width=70;height=70;mode=crop)




.png;maxWidth=300;quality=90)






